Introduction

The global medical device industry is a rapidly evolving field marked by constant innovation and technological advancement. As these medical devices continually evolve, ensuring their safe and timely delivery to their destination becomes a priority and more challenging. The process of shipping medical devices is intricate, encumbered by strict regulations, rigorous packaging requirements, and a complex array of documentation. This article can give you a basic understanding of these complexities and help you begin the labyrinth of shipping for medical devices. We highly recommend you to consult with professionals.
*Disclaimer: This article was published on November 15, 2024. Please make sure to check the latest updates on regulations and requirements for both importing and exporting countries as you prepare for the shipment.
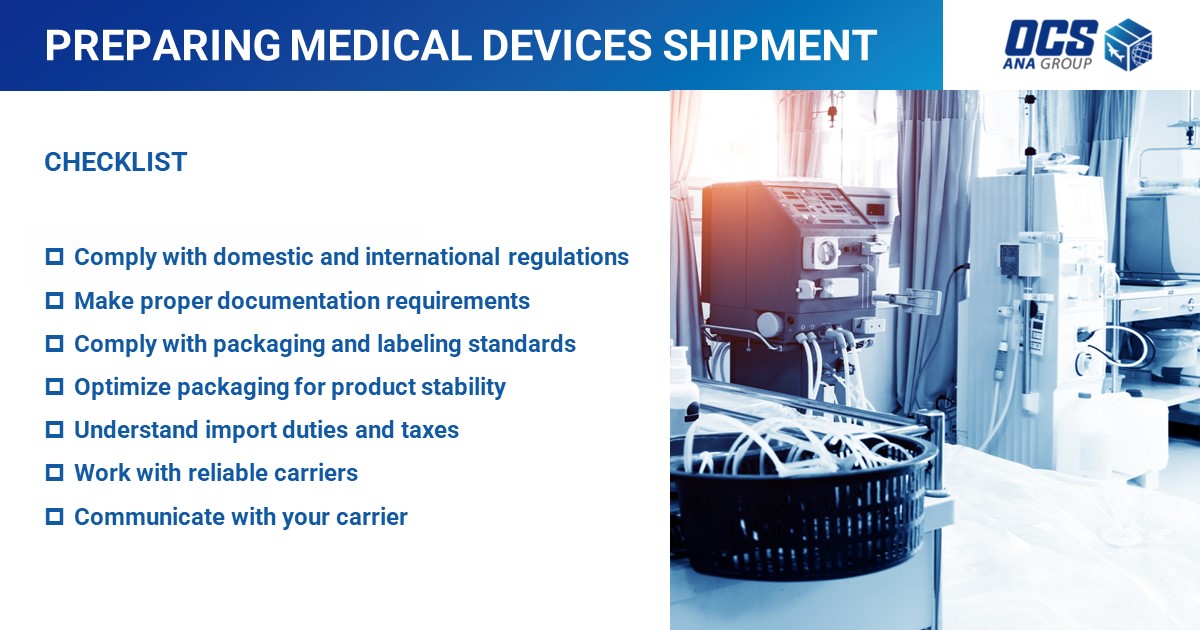
Understanding the Regulations
- Domestic and International Regulatory Bodies
The shipping of medical devices is overseen by a variety of regulatory bodies, both domestically and internationally.
In the United States, the Food and Drug Administration (FDA) regulates the import and export of medical devices (link). The FDA classifies devices into three categories (Class I, II, and III) based on their potential risks to the patient, with Class III devices subject to the most stringent regulations.
In Europe, the European Union's Medical Device Regulation (MDR) governs the shipping of medical devices (link). The MDR has recently undergone significant changes, making it essential to stay abreast of the latest requirements.
Regulations differ significantly from country to country, so it's crucial to understand and comply with the guidelines of both the shipping origin and destination countries. Non-compliance can result in legal issues, costly penalties, and significant shipment delays.
- Staying Updated with Regulatory Changes
Regulations are fluid and can change rapidly in response to technological advancements, public health crises, and other factors. Therefore, staying updated with the latest regulations is critical. This can be achieved by subscribing to updates from regulatory bodies, attending industry conferences and seminars, or consulting with legal and logistics experts.
Packaging Requirements
- Protecting Device Integrity
The proper packaging of medical devices helps maintain the device integrity during transit. The packaging should be robust enough to protect the device from physical damage, temperature fluctuations, and contamination. Different types of medical devices require different packaging solutions. For instance, delicate or sensitive equipment might require thermo-regulated packaging to protect against temperature extremes. Sterile devices, on the other hand, may require barrier packaging to prevent microbial contamination.
- Complying with Packaging Standards
In addition to protecting device integrity, packaging must also comply with international standards. For example, ISO 11607 specifies the requirements for materials and systems used for the packaging of terminally sterilized medical devices. Compliance with such standards not only ensures device safety but also helps to avoid regulatory issues.
Documentation
- Essential Shipping Documents
Proper documentation is also one of the important aspects of shipping medical devices. Essential shipping documents include commercial invoices, packing lists, and shipping labels. The Air Waybill (AWB) or also known as Courier Waybill (CWB) by OCS, in particular, must be accurately filled as it serves as the contract of carriage between the shipper and the carrier.
- Additional Documentation for Regulated Devices
For regulated medical devices, additional documentation is often required. This can include product classifications, licenses, permits, and certificates of compliance. Each country has its own specific requirements for documentation, so it's important to do the research and compile all necessary documents before shipping.
Failure to provide correct and complete documentation can result in shipment delays, penalties, or even confiscation of the shipment by customs authorities.
Conclusion
The process of shipping medical devices is a complex journey that requires a comprehensive understanding and efficient management. From finding the ever-evolving regulatory landscape to ensuring robust packaging and meticulous documentation, every step is important in ensuring the safe and timely delivery of these life-saving devices.
Despite these complexities, staying knowledgeable and adhering to best practices can help you successfully prepare for medical device shipment. Be sure to always seek professional advice or consider partnering with a logistics provider who are experienced in medical device shipping to ensure compliance and efficiency.
With careful planning and execution, OCS can be a reliable and effective solution for delivering medical devices worldwide. With the right knowledge and resources, you can ensure that these critical devices reach their destinations safely, securely, and on time.
For more information
■ About IEX Customize: Read more
■ OCS Global Network: Office locations
■ Case Study: (TBD)




.jpg)


.jpg)
.jpg)


